Abstract
Introduction: Alternative Splicing (AS) plays a pivotal role in regulating cellular processes in both normal and malignant cells. Previous studies indicated mutations in the spliceosome complex, such as SF3B1, can cause aberrant AS in acute myeloid leukemia and multiple myeloma (MM) patients. However, there is also a known interaction between AS and the DNA damage response in other cancer types, which we investigate here.
Materials and Methods: RNA-sequencing data of newly diagnosed MM patients (n=598) from the MMRF CoMMpass dataset were utilized to generate whole transcriptome AS profiles. For each patient, RNA-seq data was aligned to Hg38 reference genome using STAR while expression level was measured by Salmon. AS profiles were established from their alignment and expression profile using SUPPA2. Splicing level was defined by Percentage of Spliced-In (PSI). UMAP plots were generated for all patients using top 20,000 AS events ranked by variation in the sample set. High-quality differential AS events between groups of patients were obtained from the following thresholds: significant splicing level difference (|PSI|>10% and P <0.05 from T-test), sufficient expression level (TPM>1), not similarly spliced (|PSI|>10% and P-value<0.05 from T-test) in normal bone marrow plasma cells (N=5). Patient samples were annotated for cytogenetic, mutation, copy number and structural variation status. Pathway analysis was conducted for differentially expressed genes (DEGs). Patients' overall survival and progression-free survival was displayed with Kaplan-Meier curves. Survival difference was measured by log-rank test and cox-regression (when other well-known risk factors were incorporated).
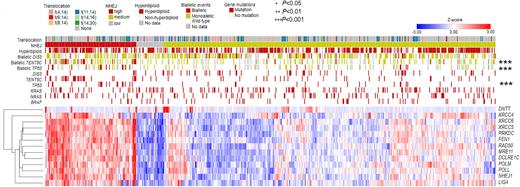
Results: We examined differential AS by defining and comparing high and low activity of key myeloma KEGG pathways including the spliceosome, proteasome, DNA repair and ubiquitination pathways. The DNA repair pathway generated significantly more (P<0.05) differential AS events (N=1,197) than the other pathways. To examine this further, we performed the analysis for the three main DNA repair mechanisms: homologous recombination (HR), non-homologous end joining (NHEJ) and microhomology-mediated end joining (MMEJ) pathways. The number of differential AS events (N=2,999) between NHEJ-high (N=121) and -low (N=36) samples was significantly more than for the other DNA repair pathways (P<0.05). NHEJ-high samples were significantly enriched for TP53 mutations (15% vs. 6%, P=2x10-5, Fisher's exact test), biallelic-TP53 events (10% vs. 4%, P=3x10-5), and biallelic-TENT5C (21% vs. 10%, P=1x10-9) events (Figure 1). Additionally, expression of LIG4 and XRCC4, encoding DNA IV ligase complex, a key component of the NHEJ pathway, correlated highly (, Spearman correlation) with the number of differential AS events. The NHEJ-high group also harbored significantly more structural abnormalities than the NHEJ-low group (mean 87 vs. 19, P=6x10-4, Mann-Whitney U test), indicating their different status of DNA stability that was highly associated with this pathway.
Survival analysis indicated that patients in the NHEJ-high group showed a significant worse progression-free survival (PFS) compared to others (Logrank statistic=7.3, P=0.007). In a multivariate analysis including other high-risk factors such as MGP-Double-Hit, t(4;14), t(14;16), TP53 mutation, 17p del, 1q gain/amp, and ISS=III, the NHEJ-high group retained significance (Hazard Ratio=1.8, P=1.6x10-5). To incorporate AS events we further applied a backward elimination approach to automatically select an optimal prognostic model from top 200 highly variant AS events ranked by variation and 7 well-known risk factors along with high NHEJ activity in PFS for the entire population. This model contained 16 significant risk factors (P<0.05, Multivariate cox regression) and 12 of them were AS events. NHEJ-high samples were significantly enriched (P<0.05, Fisher exact test) for eight of the AS events that were associated with poor prognosis.
Conclusion: AS frequency is associated with the NHEJ pathway in MM. NHEJ activity is also associated with DNA instability through increased number of structural rearrangements and is a high-risk factor.
Figure 1. High expression of the NHEJ pathways is associated with TP53 and TENT5C abnormalities.
Disclosures
Walker:Bristol Myers Squibb: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal